boron number of protons electrons and neutrons|How Many Protons, Neutrons and Electrons Does Boron Have? : Pilipinas Boron is the 5th element in the periodic table and has a symbol of B and atomic number of 5. It has an atomic weight of 10.810 and a mass number of 11. Boron has five protons . Citigold offers exclusive privileges and offers such as cash rewards, global banking and lifestyle privileges and more. Get up to S$6,626 when you join Citigold. Citigold Privileges and Offers. Only for you. Only with Citigold. Get Started. Explore the .
boron number of protons electrons and neutrons,Element Boron (B), Group 13, Atomic Number 5, p-block, Mass 10.81. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.
How Many Protons, Neutrons and Electrons Does Boron Have?The mass of an atom relative to that of carbon-12. This is approximately the .
The mass of an atom relative to that of carbon-12. This is approximately the .Boron is the 5th element in the periodic table and has a symbol of B and atomic number of 5. It has an atomic weight of 10.810 and a mass number of 11. Boron has five protons .
Wayne Breslyn. 780K subscribers. Subscribed. 233. 29K views 4 years ago. In this video we’ll use the Periodic table and a few simple rules to find the protons, .Name: Boron. Symbol: B. Atomic Number: 5. Atomic Mass: 10.811 amu. Melting Point: 2300.0 °C (2573.15 K, 4172.0 °F) Boiling Point: 2550.0 °C (2823.15 K, 4622.0 °F) Number of Protons/Electrons: 5. Number of . The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after the element. To find the number of neutrons, subtract the element’s atomic number .
Number of electrons in shells. Thus, 1st shell can hold 2 electrons. 2nd shell can hold 8 electrons. 3rd shell can hold 18 electrons. 4th shell can hold 32 electrons. Now the atomic number .
As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron . In order to be neutral, an atom must have the same number of electrons and protons. Atoms—and the protons, neutrons, and electrons that compose .

Boron atomic number 5 has five electrons in its ground state. Commonly Boron will lose 3 electrons leaving 2 electrons in its most common ionic form. The atomic .
Electrons always provide a negative charge. It is expressed by e –. The charge of electrons is –1.609 × 10 –19 coulombs and the relative charge is –1. That is, the charge of an electron is equal to that of a proton but the opposite. Try the Proton Neutron Electron Calculator and get instant results for any element.The relative masses of atoms are reported using the atomic mass unit ( amu ), which is defined as one-twelfth of the mass of one atom of carbon-12, with 6 protons, 6 neutrons, and 6 electrons. 1.2: Atomic Structure - The Nucleus CC BY-SA 4.0. Atoms are comprised of protons, neutrons and electrons. Protons and neutrons are found in the nucleus .
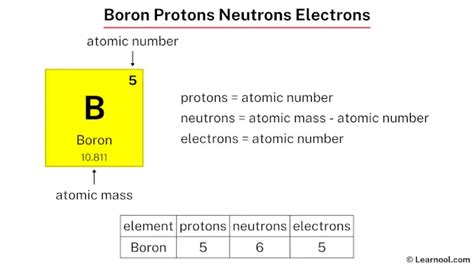
boron number of protons electrons and neutrons How Many Protons, Neutrons and Electrons Does Boron Have?The mass number (\ (A\)) of an atom is the total number of protons and neutrons in its nucleus. The mass of the atom is a unit called the atomic mass unit \ (\left ( \text {amu} \right)\). One atomic mass unit is the mass of a proton, or about \ (1.67 \times 10^ {-27}\) kilograms, which is an extremely small mass.The mass of a proton or a neutron is about 1836 times greater than the mass of an electron. Protons and neutrons constitute the bulk of the mass of atoms. . boron \(\ce{^{10}_5B}\) 5: 5: 5: 10.0129: 19.9 \(\ce{^{11}_5B}\) 5: 5: 6: 11.0093: 80.1: carbon . Determine the numbers of protons, neutrons, and electrons in one of these iodine . Number of Neutrons = Mass Number - Number of Protons = 1 - 1 = 0. For zinc, the atomic weight is 65.39, so the mass number is closest to 65. Number of Neutrons = 65 - 30 = 35. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element. Lithium has 3 protons, 4 neutrons and 3 electrons. 4. Beryllium has 4 protons, 5 neutrons and 4 electrons. 5. Boron has 5 protons, 6 neutrons and 5 electrons. 6.

Calculate numbers of protons, neutrons, and electrons by using mathematical expressions (1-3): p = 11 (1) n = 23 - 11 = 12 (2) e = 11 - 0 = 11 (3) Alternatively, you can also calculate the atomic number, atomic mass, and charge. Choose your element. Let's assume that it is the sulfide anion.
The number of protons can be found by knowing the atomic number of that atom. Number of Protons in Boron = Atomic number of Boron = 5. Number of Neutrons in Boron. The number of neutrons can be found by subtracting the atomic number from its atomic mass. Number of Neutrons in Boron = Atomic mass of Boron .
boron number of protons electrons and neutrons|How Many Protons, Neutrons and Electrons Does Boron Have?
PH0 · How to find the Number of Protons, Electrons, Neutrons for Boron
PH1 · How to Find the Number of Protons, Neutrons, and
PH2 · How many electrons does boron have?
PH3 · How Many Protons, Neutrons and Electrons Does Boron Have?
PH4 · Chemical Elements.com
PH5 · Boron (B)
PH6 · Boron
PH7 · 2.6: Protons, Neutrons, and Electrons in Atoms